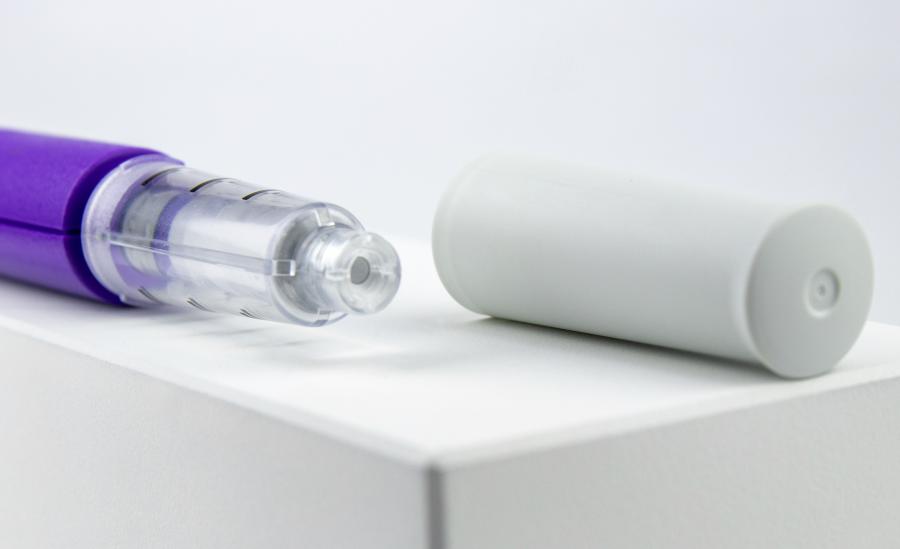
In collaboration with Shaily Plastics, a long-time customer based in India, IDC's medical product design team in the UK has developed a fixed-dose injection pen suitable for providing a variety of different treatments. In short, this injection pen meets the quality requirements and is affordable. Shaily can be mass-produced and sold to major pharmaceutical companies to make it its own drug delivery device.
The product should meet the administration requirements of various pharmaceutical treatments, such as teriparatide, human growth hormone (HGH), follicle-stimulating hormone (FSH), glucagon-like peptide 1(GLP-1), etc. Each drug delivery pen can provide the user's daily medication requirements for more than 28 days.
Having worked with IDC in the UK to develop similar products, Shaily is well versed in IDC's ability to design and develop new patent-eligible products. This is a very important consideration because the history of drug delivery device development is riddled with patent protection challenges.
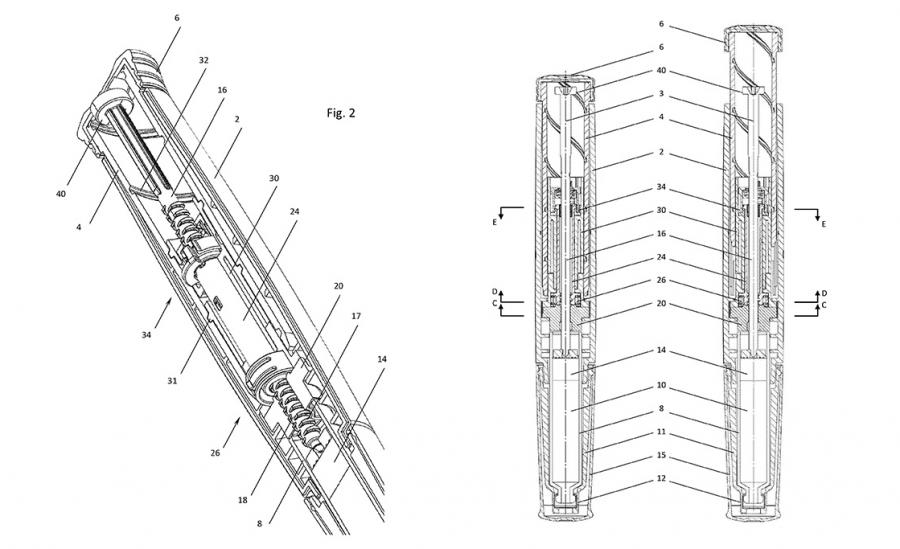
To ensure that the best design solution is created for users, IDC UK engineers and designers work closely together to provide users with a rigorous, fast and simple dosing system. The safety of drug delivery is the focus of this design. This drug delivery device has a complete fail-safe mechanism and some design features to prevent user misuse. For this reason, IDC in the United Kingdom has developed a "pull-rod drug delivery" mechanism to ensure that the drug is only output when the device is used correctly.
Throughout the development process, IDC Models was responsible for making prototypes. Rapidly developed SLA prototypes are valuable for refining structural forms and testing structures in actual development. IDC is responsible for the entire product development lifecycle and provides testing and validation processes for partners across Europe. IDC provides institutions with full regulatory documentation and full traceability to obtain medical certification in Europe and the United States. This includes FDA and ISO 11608 quality standards.
These injection pens are manufactured almost entirely in medical grade plastic in Shaily's world-leading factory, and combine PC,PCM,PC / ABS and glass filling materials. IDC's unique structural patents and Shaily's most high quality manufacturing make the Axiom injection platform commercially successful and still successful.
"We have selected IDC as our partner for many of our current drug delivery device projects, each time they have exceeded our expectations and provided excellent design development services for complex technical problems in a short period of time. Their ability to develop new intellectual property for our business in a challenging market is something we value highly. I strongly recommend working with IDC on any medical device design and development project."
--Amit Sanghvi Shaily




The copyright of this work belongs to 英国IDC产品设计. No use is allowed without explicit permission from owner.

New user?Create an account
Log In Reset your password.
Account existed?Log In
Read and agree to the User Agreement Terms of Use.

Please enter your email to reset your password
The picture is a little burnt.
That's great.
good effect
Perfect
it can be